Abstract

Health is not just defined as the absence of disease, but also as the state of physical, emotional, and social well-being. Sickle cell anemia (SCA)-children have poor health-related quality of life (HRQL), principally because of poor physical functioning and severe pain. Improving HRQL has become an important goal of medical care. We present here the results of HRQL at 1 year in the DREPAGREFFE trial.
DREPAGREFFE, a French prospective, Mendelian randomized trial with 2 arms (transfusions/transplantation) defined by random-availability of a HLA-matched sibling, enrolled SCA-children younger than 15, regularly transfused for history of abnormal velocities by TCD, with at least one non-SCA sibling, and parents agreeing to HLA-typing and transplantation. Conditioning consisted of intravenous busulfan, cyclophosphamide and rabbit anti-thymocyte globulin. HbS was maintained below 30% when transfused. Between 12/2010 and 6/2013, 67 SCA-children (7 with stroke history) were enrolled. Thirty-two had a genoidentical donor and were transplanted, while 35 (no donor) were included in the transfusion arm. No stroke was observed during the 12-months follow-up. All transplanted patients successfully engrafted; three acute-GvHD (grade≥II) were observed, but none chronic-GvHD. All patients were alive at 1-year. Transplanted patients had the same hemoglobin electrophoresis as their donor and significantly lower median [IQR] cerebral velocity, and more had normalized velocities compared to the transfusion arm. However, no significant difference in cognitive performance was observed between the 2 arms at 1-year.
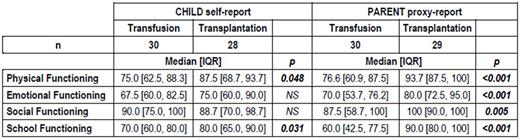
HRQL at 1-year, was one of the trial secondary outcome. Data were collected using the PedsQL 4.0 Generic Core Scale, a generic HRQL questionnaire with a child self-report for aged 5 through 18 and a parent proxy-report for children aged 2 through 18. The scale contains 23 items assessing physical (8), emotional (5), social (5), and school functioning (5). Four composite scores (physical, emotional, social, and school functioning) were calculated. Scores ranged from 0 to 100, with the higher scores indicating higher HRQL.
At 1 year, the child self-report showed significantly better scores in transplanted than transfused children for physical health (p=0.048) and school functioning (p=0.031) (Table). Significant differences were observed for items describing "hurt" (p=0.006) and "low energy" (p=0.021), and those about missing school due to "not feeling well" (p=0.029) or "going to the hospital" (p=0.001). The parent proxy-report showed highly significant better scores in transplanted children for all scales: physical (p<0.001), emotional (p<0.001), social (p=0.005) and school functioning (p<0.001).
We show here that SCA-children have better physical health and school functioning after transplantation than on chronic transfusion, and that the differences observed concern the hurt, energy and school missings, while parents report strong differences for all items. Improvement of HRQL has been previously reported after transfusions at 3-years in a randomized trial (Silent Infarct Transfusion) (DeBaun 2014), and after transplantation in non-randomized study (Bhatia 2015), but this is the first randomized trial comparing transfusion to transplantation in SCA. It has been shown that parents usually report worse HRQL for their child than the child self-reports (Panepinto 2010) . This disagreement between parents and children has been reported to be related to parents' distress or disease severity. In contrast, in the present trial, parents of transplanted children reported a better HRQL than their child, suggesting better parental well-being, which is probably related to the better HRQL observed in their child and to anticipation of a better future.
Peffault De Latour: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Brousse: Add Medica: Membership on an entity's Board of Directors or advisory committees. Socié: Alexion Pharmaceuticals, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal